Understanding the complex world of organic chemistry is a pivotal step for students aiming to ace their A levels H2 chemistry exams. The Ace Scorers Enrichment Centre is dedicated to helping students unravel the mysteries of organic compounds, particularly alkanes, aldehydes, ketones, and carboxylic acids. This article delves into the nuances of carbonyl compounds and provides insights into why certain molecules behave differently during chemical reactions, specifically oxidation to help students master the Intricacies of Carbonyl Compounds and success in chemistry.
What are Carbonyl Compounds for H2 Chemistry?
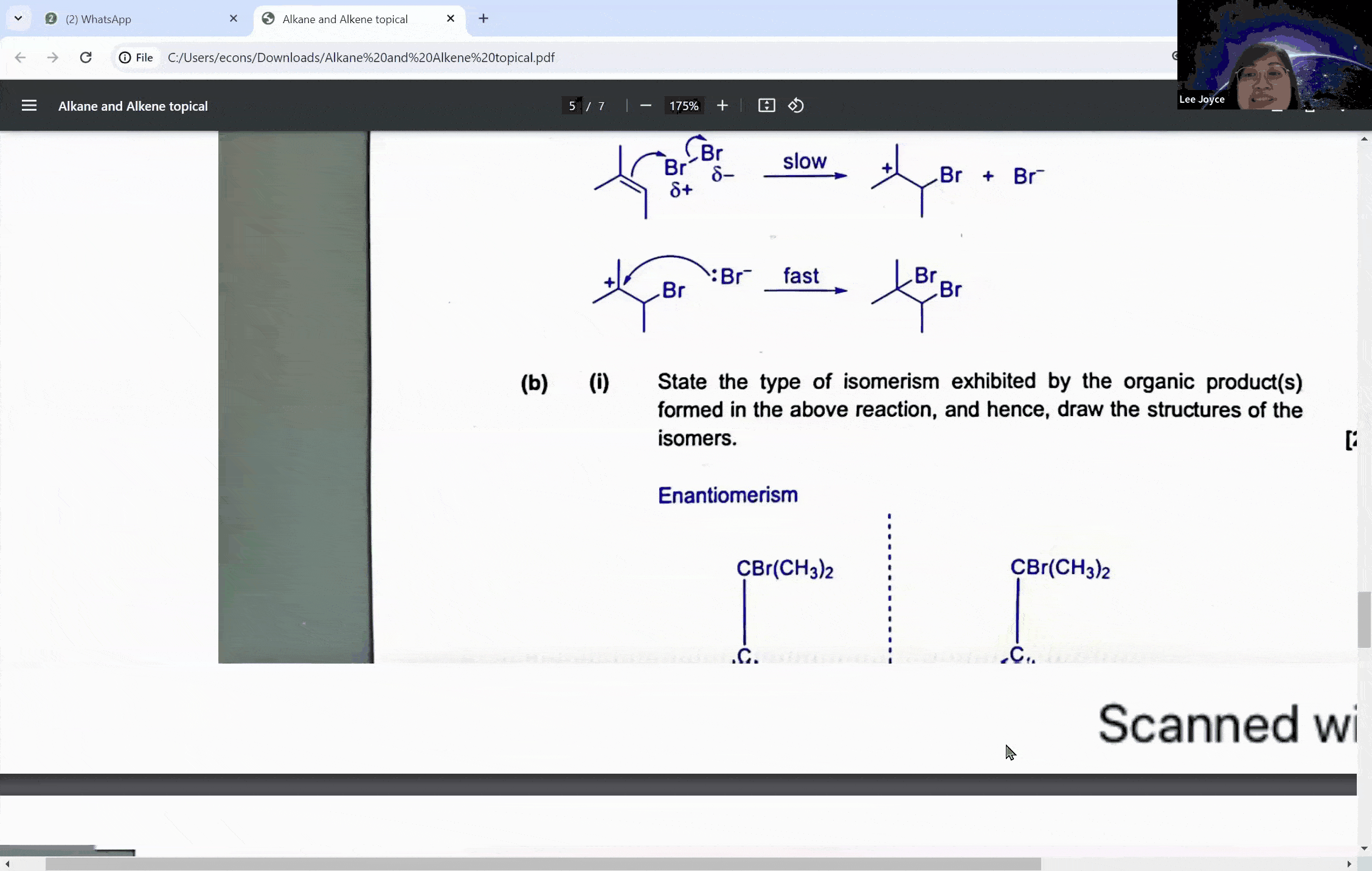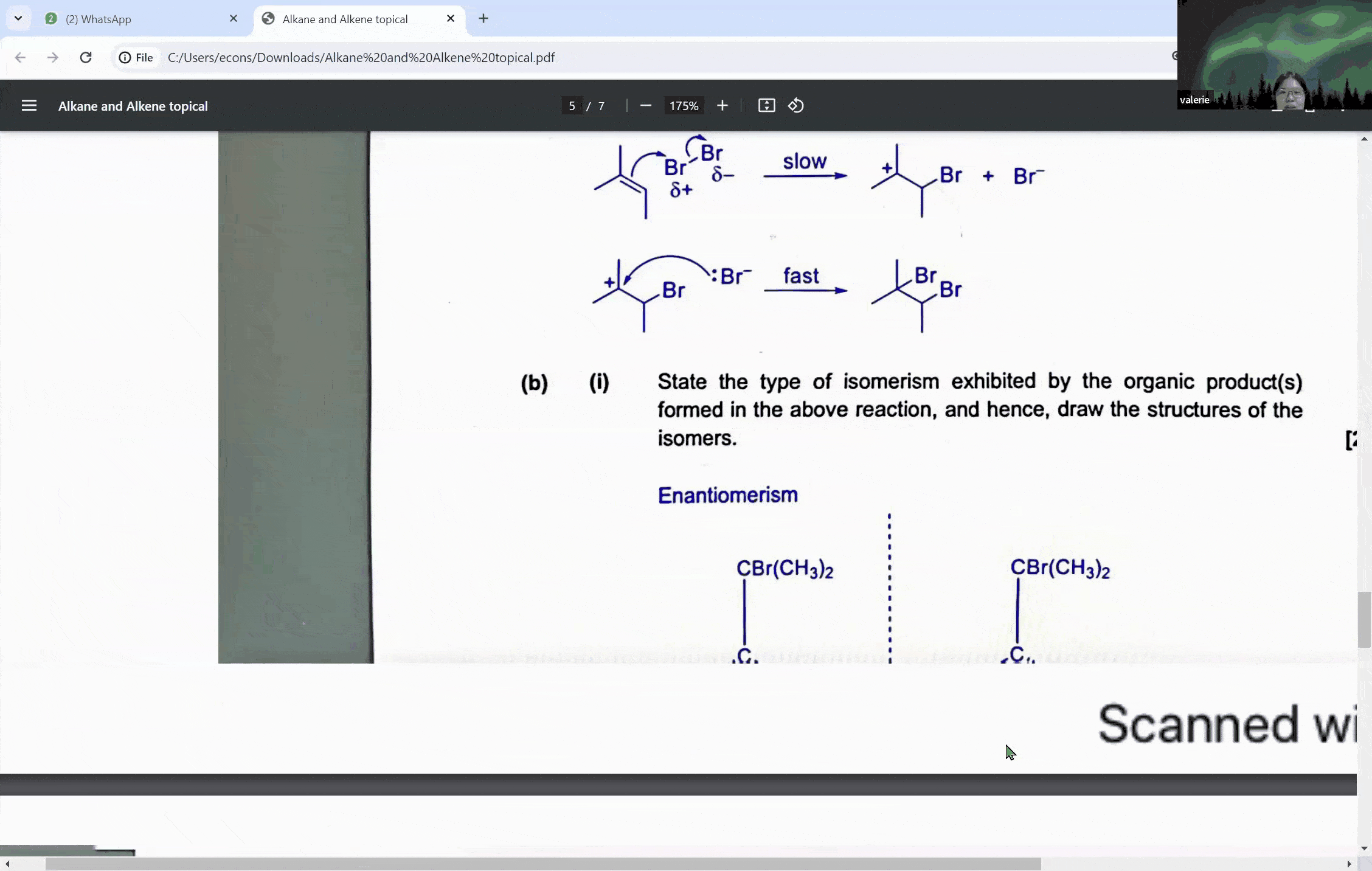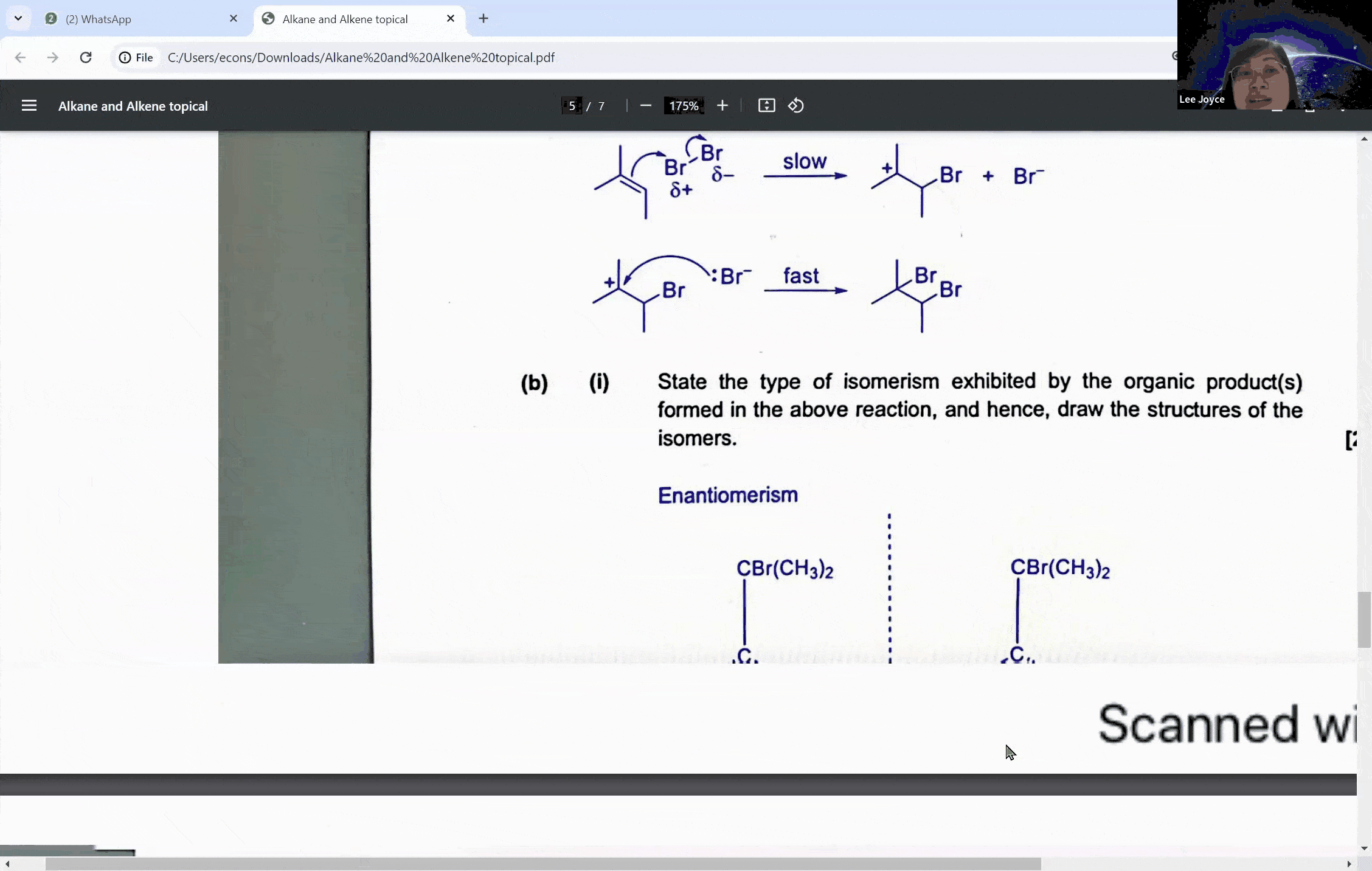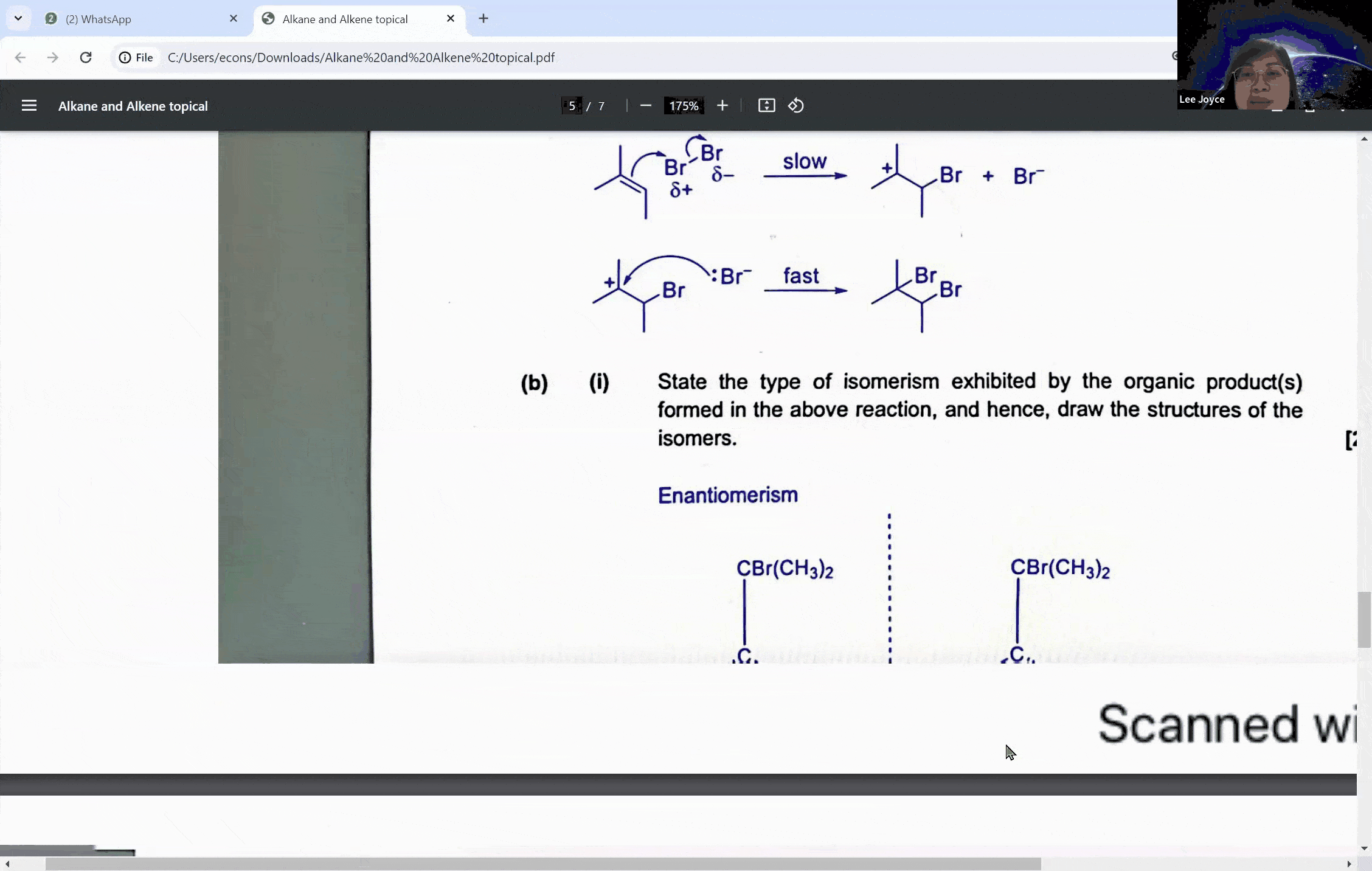
Carbonyl compounds are a central topic in A levels chemistry, characterized by the presence of a carbon-oxygen double bond (C=O). This functional group is the defining feature of both aldehydes and ketones, which, despite their similarities, exhibit distinct reactivity patterns, especially when it comes to oxidation. The ability to distinguish between these compounds is not just a matter of rote memorization; it is a skill that can set the foundation for students to achieve an A in their exams.
Aldehydes are carbonyl compounds with at least one hydrogen atom attached to the carbonyl carbon, whereas ketones boast two alkyl groups. This structural difference is not trivial—it is the key to understanding their differing reactivities. Aldehydes can be readily oxidized to form carboxylic acids because they contain a hydrogen atom that can be lost during the oxidation process. Ketones, on the other hand, lack this hydrogen, rendering them resistant to oxidation under similar conditions.
How we can help A Levels H2 Chemistry understand better
The Ace Scorers Enrichment Centre emphasizes the importance of visualizing molecular structures to help students get A grades. By recognizing that a ketone cannot undergo oxidation due to the absence of a hydrogen atom, students can approach their A levels H2 chemistry exams with confidence. This understanding is further reinforced through the use of modern educational tools such as iPads and interactive applications like OneNote, which allow for a dynamic and engaging learning experience.
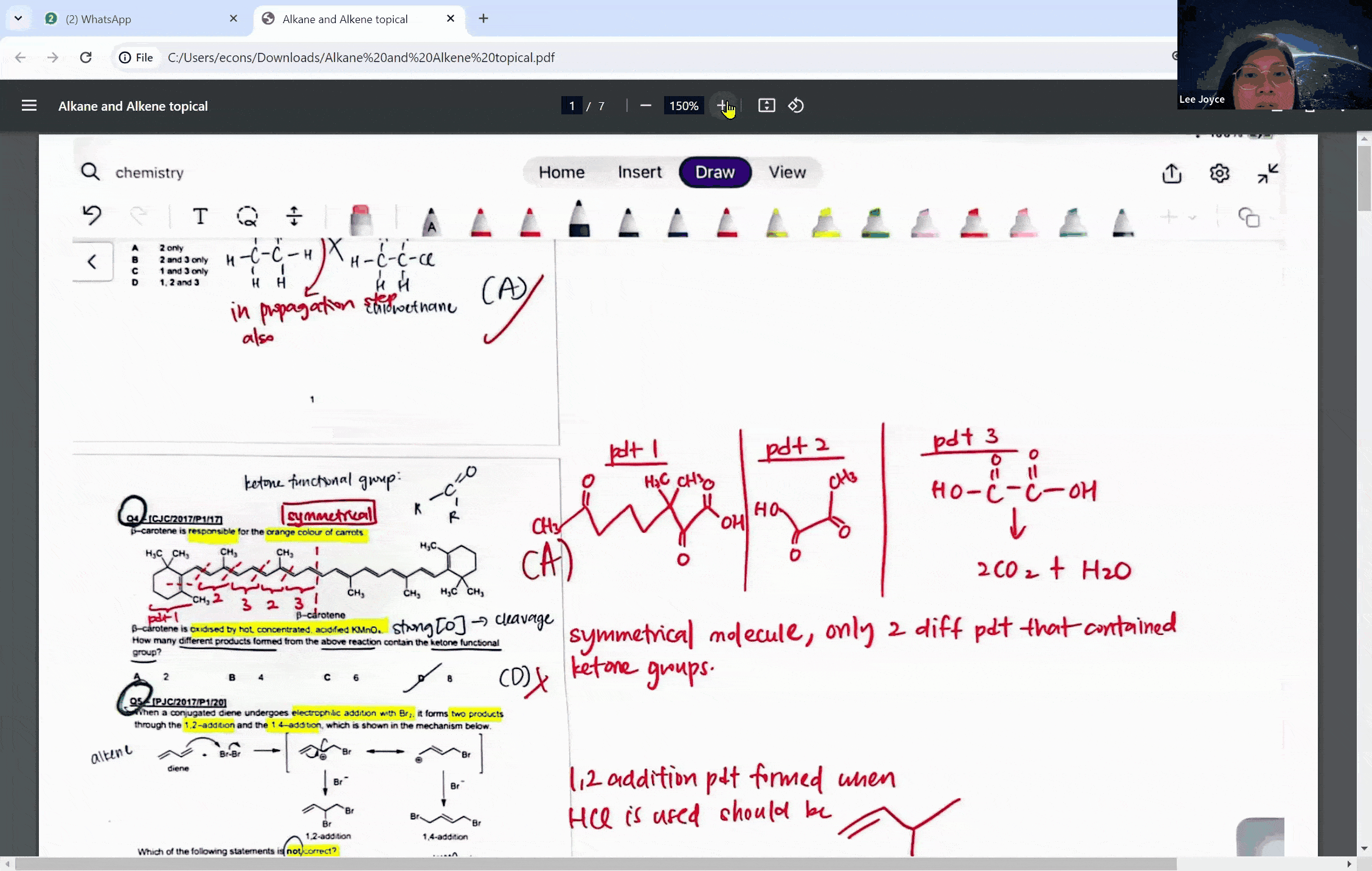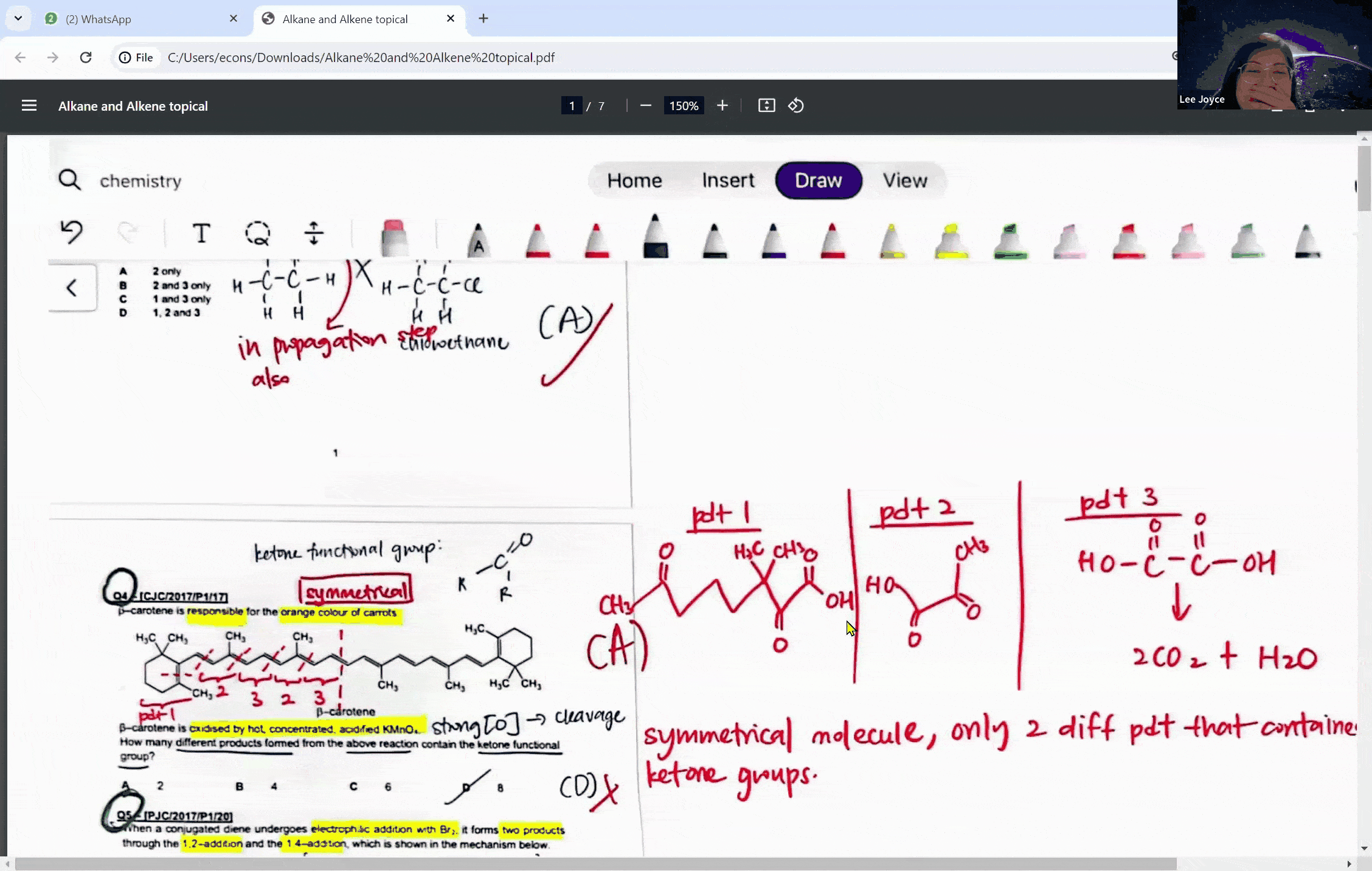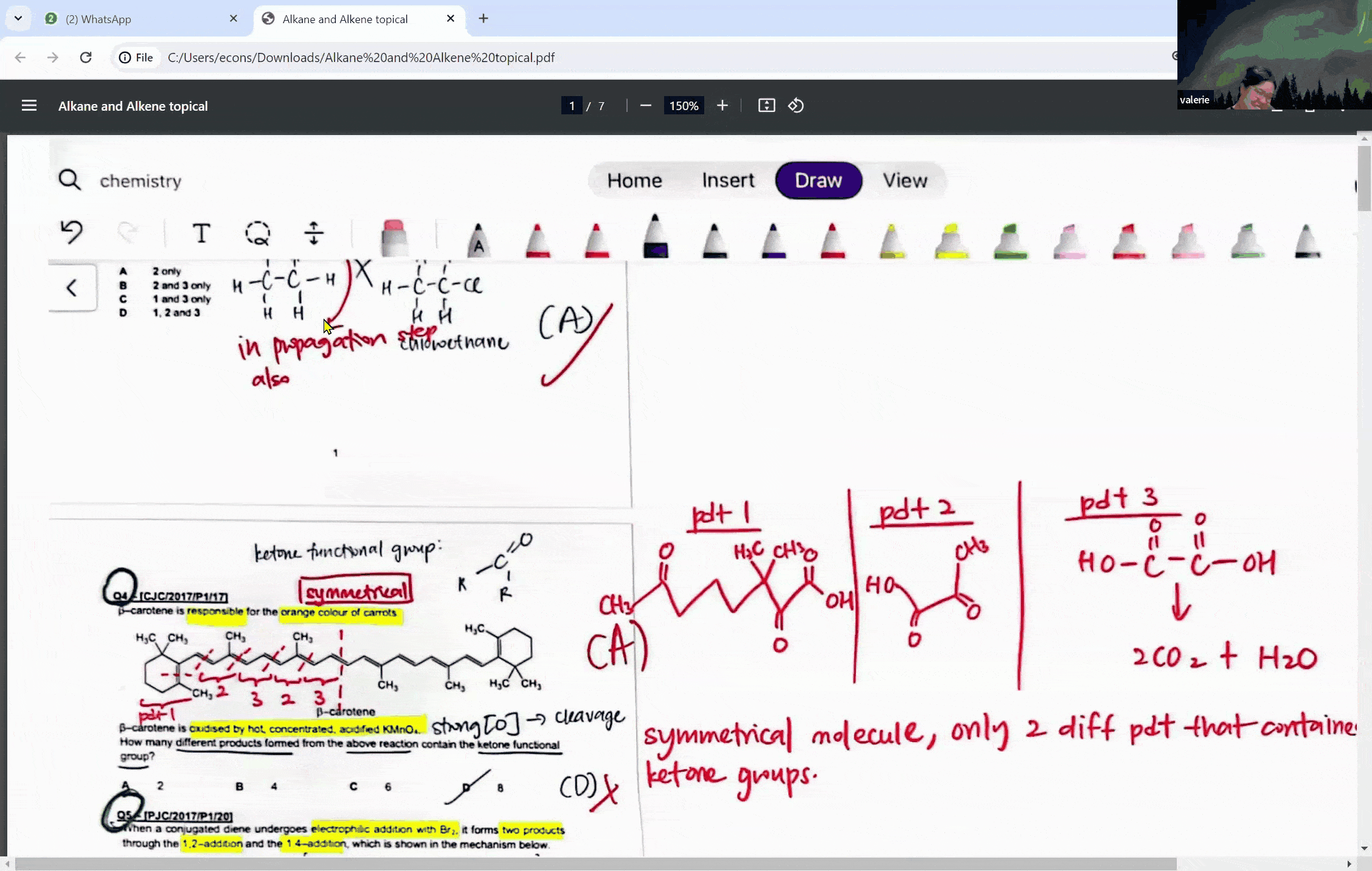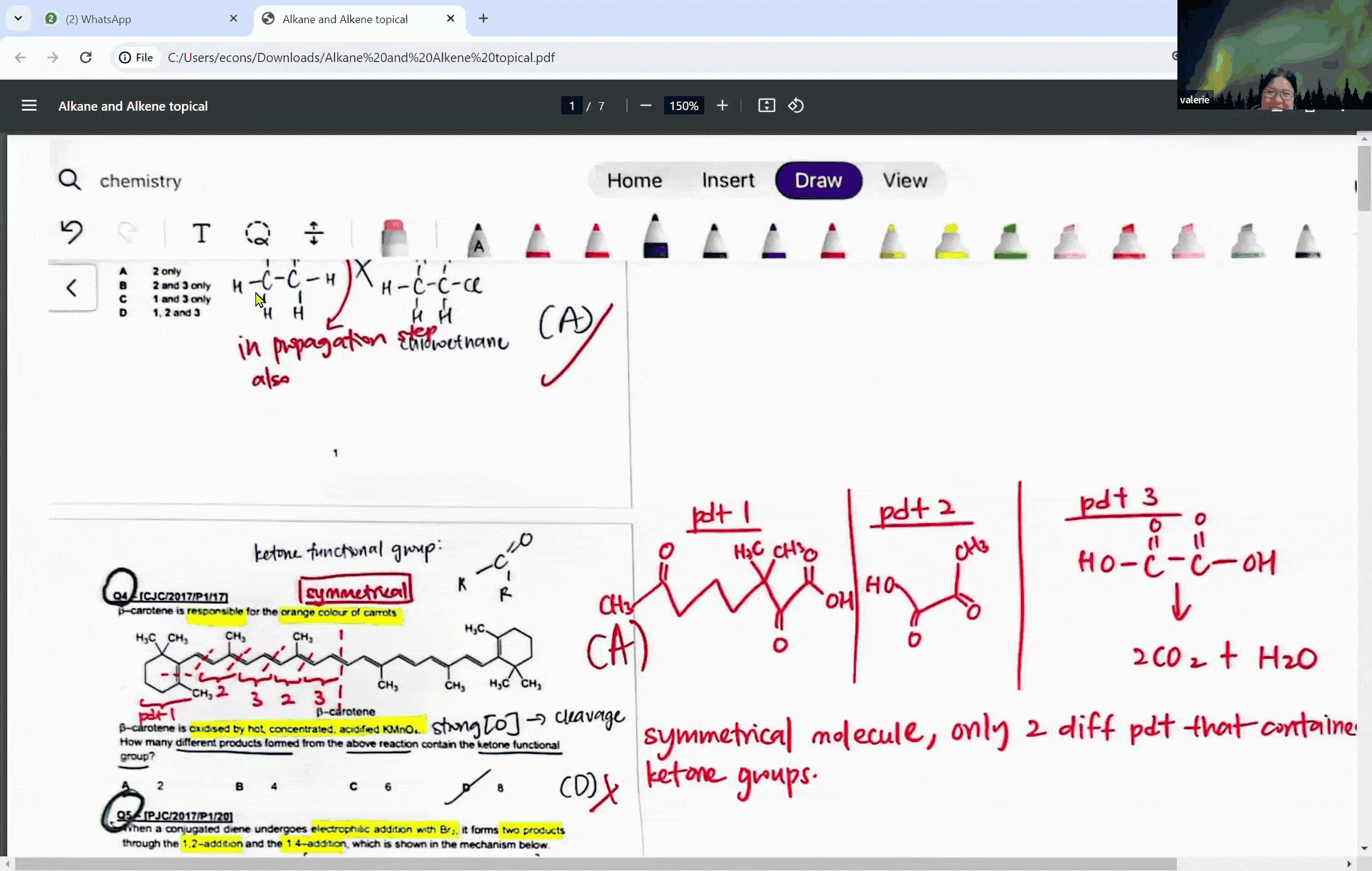
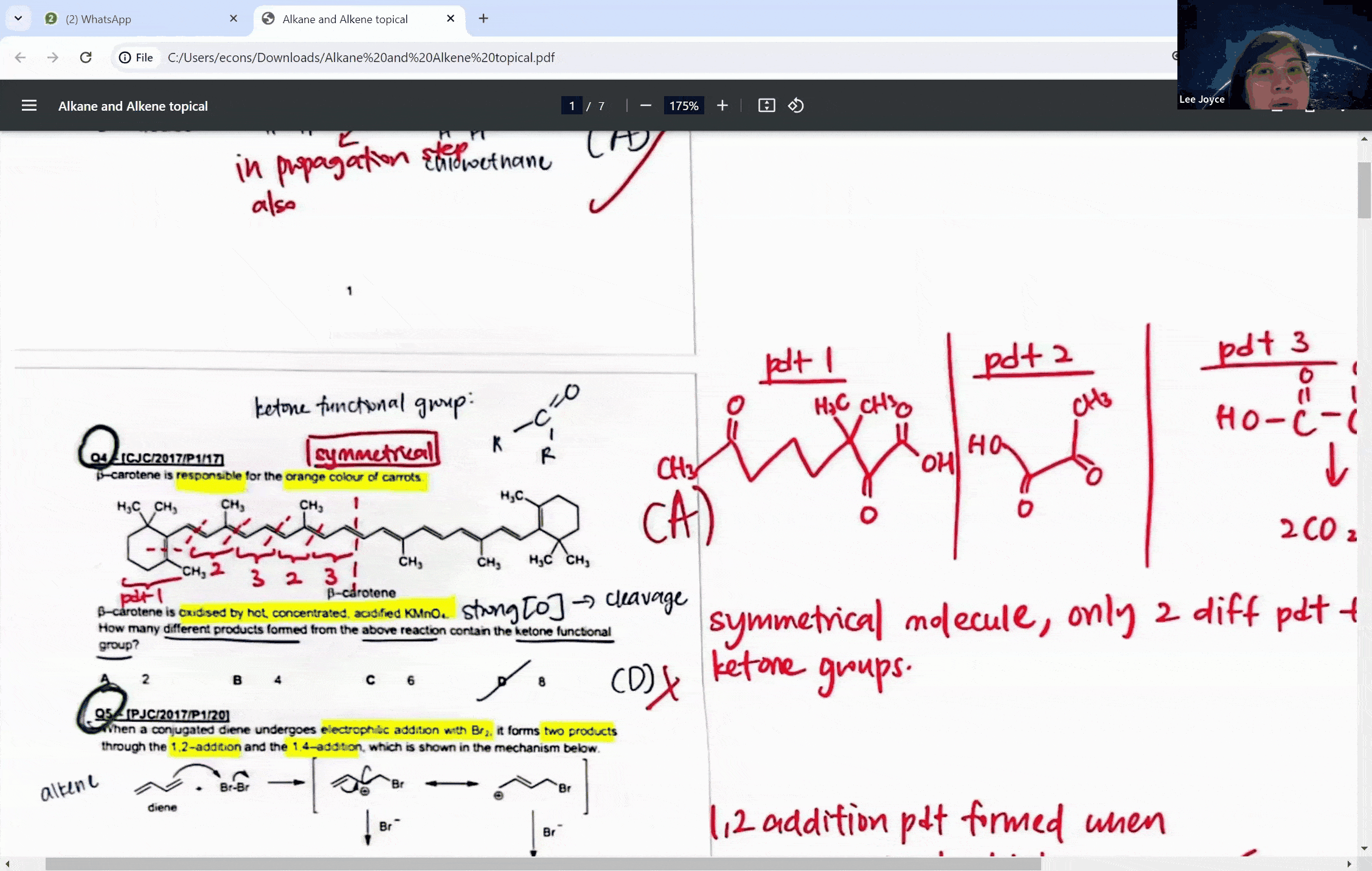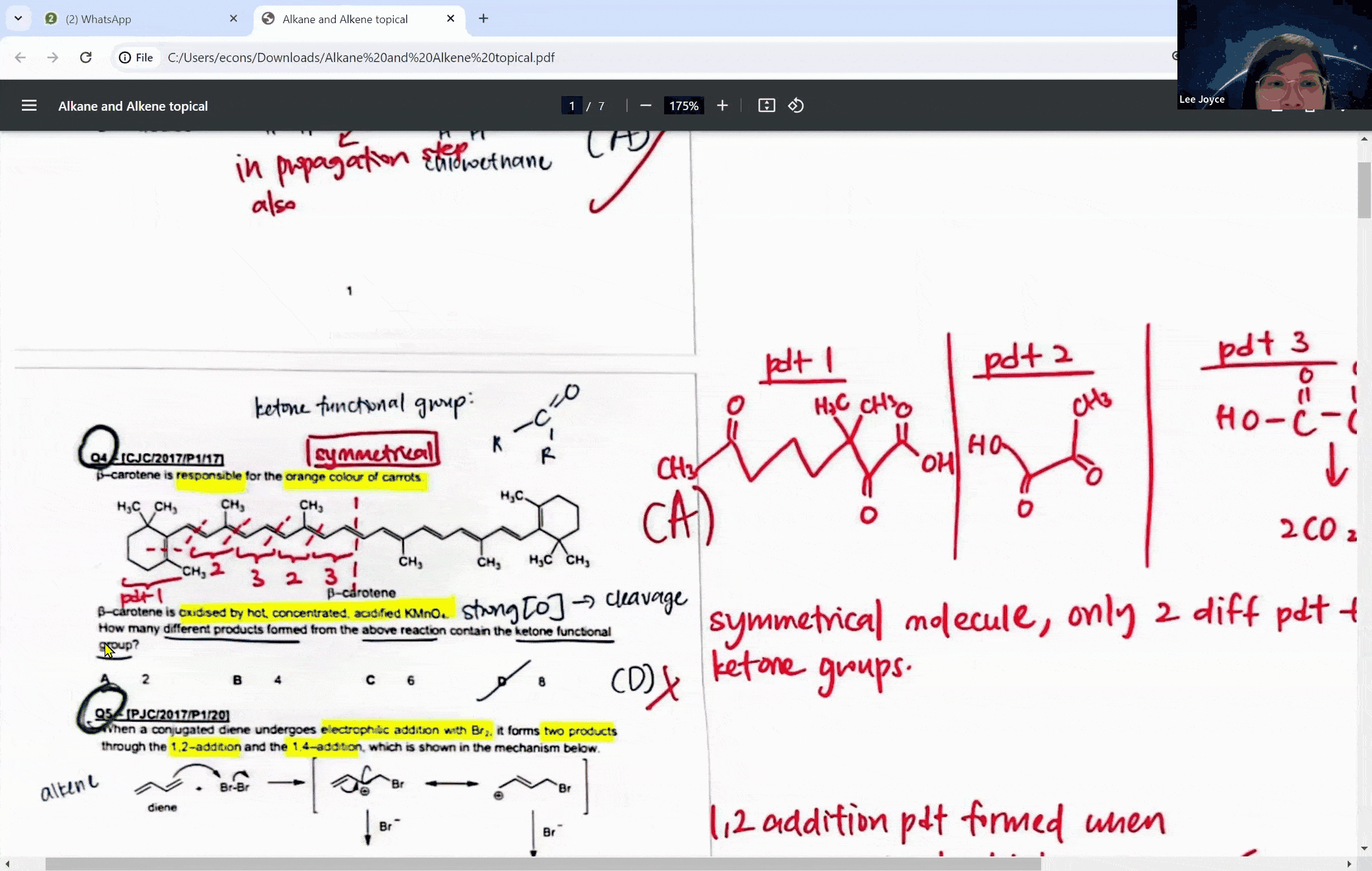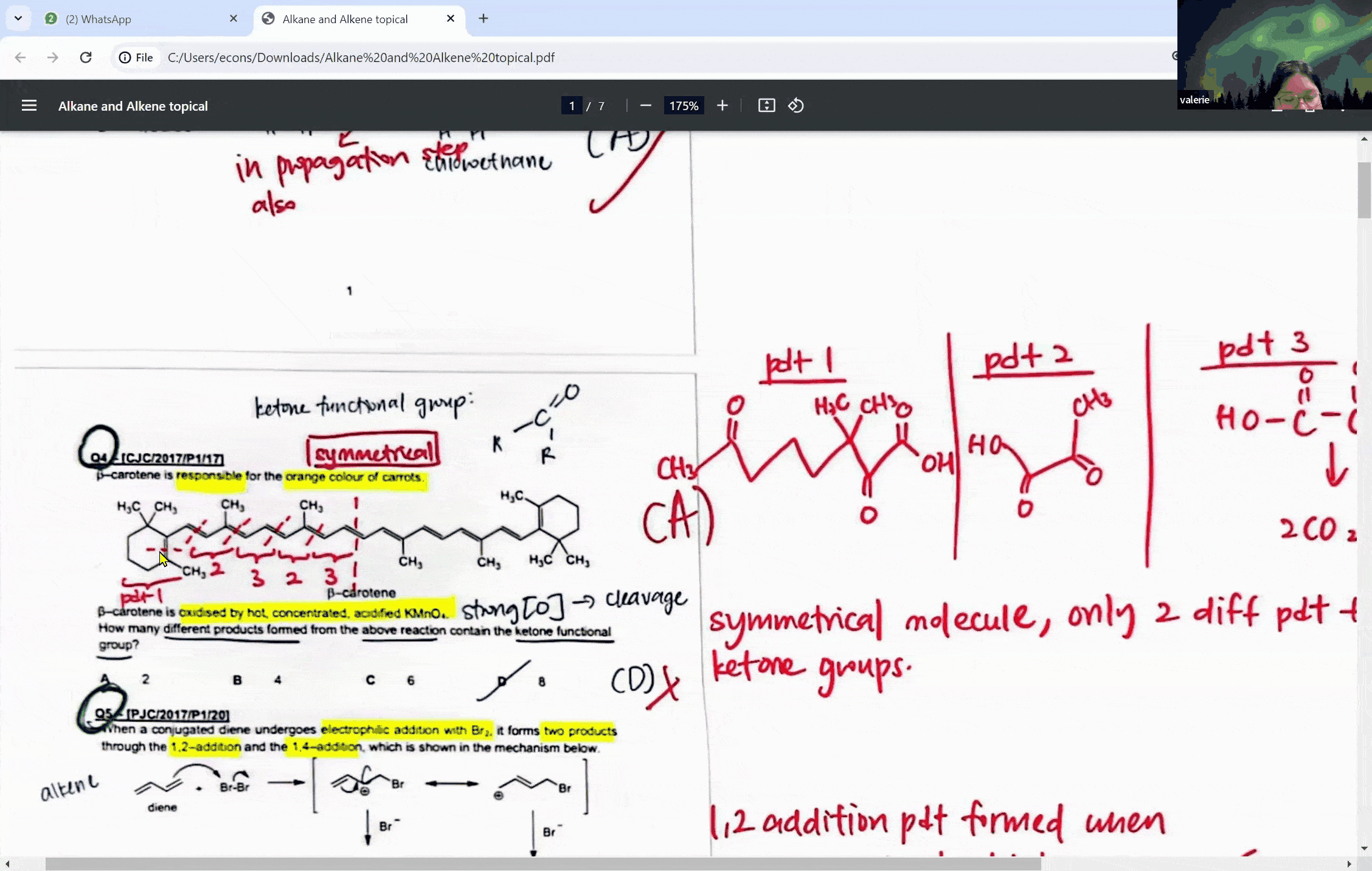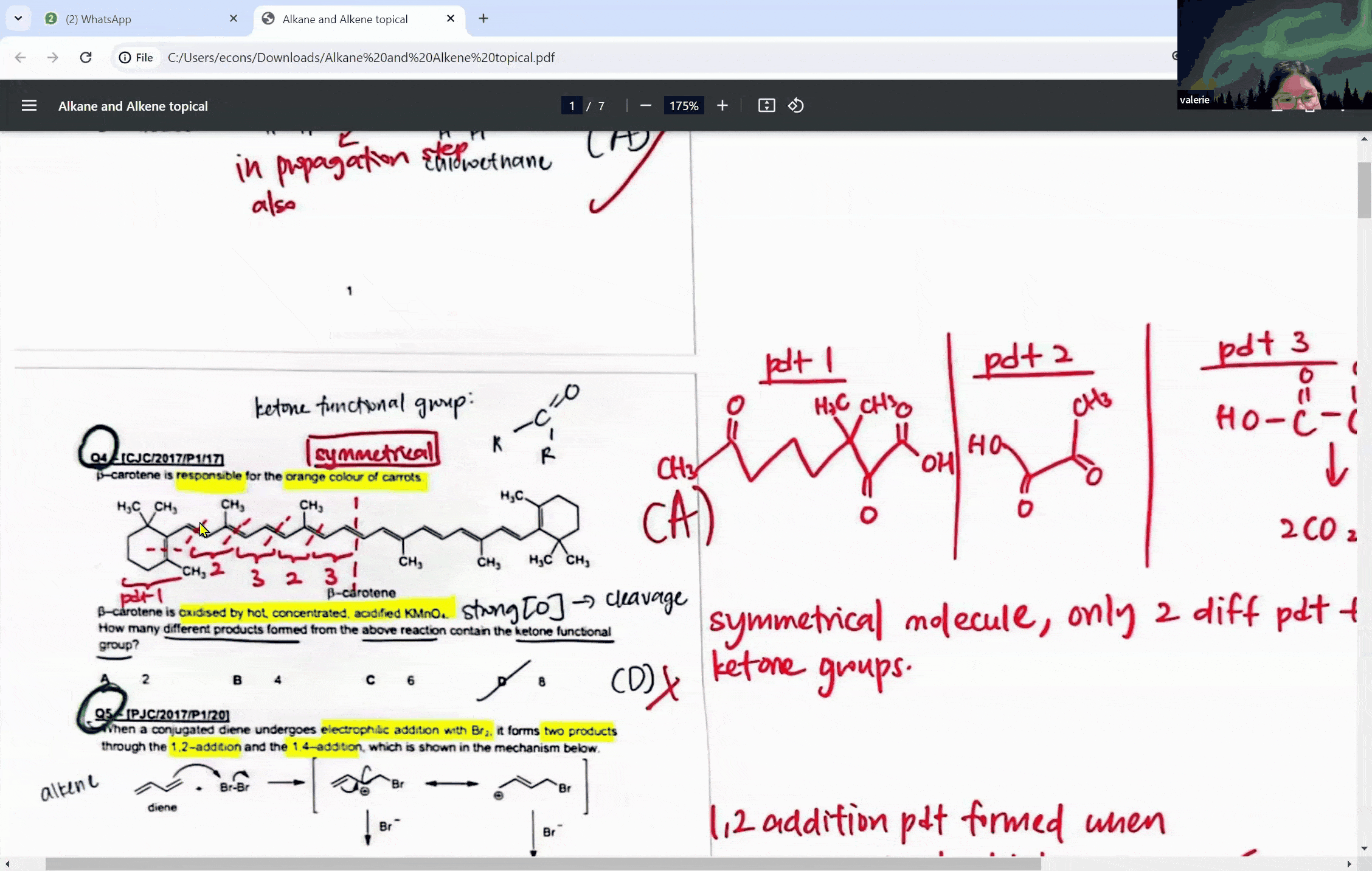
During a typical session at the enrichment centre, students might encounter a question involving the cleavage of a symmetrical molecule. Here, the concept of symmetry plays a crucial role. If a molecule is symmetrical, the products of cleavage will be identical on both sides of the symmetry line. This understanding can prevent students from overestimating the number of different products formed in a reaction, a common pitfall that can lead to incorrect answers.
For instance, when a symmetrical ketone is cleaved during oxidation, students might mistakenly count each side of the cleavage as a separate product. However, with proper guidance, they learn that the identical parts combine to form a single product. This level of discernment is what the Ace Scorers Enrichment Centre strives to instill in its students, ensuring they are well-prepared for their exams.
Conclusion
In conclusion, the journey to mastering O levels chemistry is paved with the intricate details of organic molecules and their reactions. The Ace Scorers Enrichment Centre stands as a beacon of knowledge, guiding students through the complexities of alkanes and carbonyl compounds. By fostering a deep understanding of the subject matter, the centre helps students get A grades, not through memorization, but through comprehension. As students continue to explore the fascinating realm of chemistry, they should ponder this: What other chemical behaviors and patterns can be unraveled through the study of molecular structure, and how might this knowledge be applied to real-world scenarios beyond the classroom?
Check out more of our videos here. https://www.youtube.com/@acescorers3110
